A way to reduce CO2 in the atmosphere
This would work and could be used to dramatically reduce CO2 in the atmosphere, it is not however politically correct, and in general is not acceptable to leftists who push the whole climate change. Nor would it be exactly cheap, but I think it would be enormously less costly than the nightmare sea level rise those pushing climate change.
So to the basic facts in the recent past.
https://www.esrl.noaa.gov/gmd/ccgg/trends/full.html
From this website:
So around 1960 we had a CO2 ppm of under 320, and we now have a value of ~ 407, and the rate of rise is going up (because humans are increasing consumption of fossil fuels).
https://en.wikipedia.org/wiki/Carbon_dioxide_in_Earth%27s_atmosphere
From this above site, the ppm levels before the start of the industrial revolution were around 280 ppm. This below graph is from the above website.
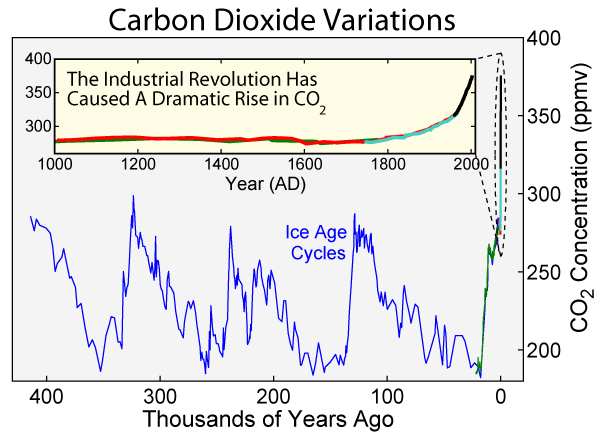
~ 200 years of heavy industrialization and buildup of carbon-dioxide Now the ppm of carbon is ~ 407, and rising at about 2 ppm per year.
In 1950 the ppm of carbon was ballpark 300, I know it was slightly less in Thomas Jefferson's time and it was pretty flat for centuries before, and we had the year without a summer not long after that, so for the sake of argument call the amount we need to move the content of the atmosphere down to arrest global warming is 100 ppm, and that the rate of rise is now on the order of 2 ppm CO2 per year.
How are we going to do that I hear you ask? Like this, and note that this sort of thing has been done by nature from time to time so this is not "unnatural" and is in fact how many fossil fuel deposits formed.
We need to have a means of conversion that converts and sequesters a minimum equivalent of 2 ppm a year to match current addition of CO2. Double that would pull us back down to 300 ppm in 50 odd years, and 25 if we are cranking out 6 ppm worth per year. 2 for each year a to stay level, 4 per year reduces the ppm by 100 ppm per 25 years. If we started in 2020, by 2045 we are back to CO2 levels of 1950.
Now follows math, if you cannot deal with it, sorry. So how many tons of CO2 is 1 ppm in the atmosphere? I am going to omit sources of commonly known things like the radius of the earth and commonly known constants from physics and chemistry.
The radius of the earth is 6371 km, the surface area of a sphere is 4*PI*R^2
So the surface area of the earth is:
510 e+6 square kilometers or 510 e+14 square meters.
The atmosphere has a pressure of 14.7 psi at sea-level, thus a pressure of 10,334 kg per square meter.
The molecular weight of air is 28.97 grams/mol the molecular weight of CO2 is 44 grams/mol
So the weight of CO2 per ppm per square meter is: 10,334 *44/(28.97*1e6)= 0.0157 kg/m^2
Or 15.7 metric tons of CO2 per square kilometer per ppm of CO2 atmospheric content.
Total of 8 e+9 metric tons of CO2 per each ppm on earth. The carbon is a mass fraction is 12/44 of the mass of CO2
To get a handle on how much surface area of the earth we need for photosynthesis, I shall assume that we can get efficiency of production of carbon to sequester roughly equal to that of sugar cane conversion of sunlight+ CO2 to O2 + sucrose+ cellulose and other carbonaceous matter at a low latitude as that is where we place the farm.
http://www.ers.usda.gov/datafiles/Sugar_and_Sweeteners_Yearbook_Tables/US_Sugar_Supply_and_Use/Table15.xls
In Hawaii (low latitude) typical production rates of pure sugar per acre of land per year is 10 short (2000 lb) tons in that they have deducted seed and note that ~ 90-100 tons of raw cane are produced per acre. A square kilometer is 247.1 acres. Thus a square kilometer can produce 10*247.1/1.1 = 2246 metric tons per square kilometer per year of pure sugar, and about ten times that of total carbonaceous material.
Then sugar (sucrose - C22H22O11) has a molecular weight of 342.3 grams per mol. Of which 264/342.2 is carbon, assume for the sake of argument this is approximately correct all the carbonaceous material generated.
So to get 1 ppm of CO2 out of the air in one year I need to produce and sequester
8E+9 x 12/44 x 342.2/264 tons of pure sugar or 2.83 e+9 metric tons of sugar.
For which I need 1.260 e+6 square kilometers of which the earth has 510. E+6
To get 6 ppm per year, I would need to pull down the CO2 back to 1950 levels in 25 years from start I need 7.56 million square kilometers of sea near the equator. Again the earth has 510 million, this is about 1.5% of the earth's surface. A circle 3100 km in diameter will do it.
I can do better by using the full weight of carbonaceous material, rather than about 1/10th, that would reduce the required area to about 1/10 or 0.76 million square kilometers, or a circle of ~ 990 kilometers in diameter.
You should get the point, nothing about this is impossible. This is possible and not really expensive in the long haul, not compared to the alternative.
All we do is build buoys that spread fertilizer, and load them with fertilizer, and bring in periodic loads of sand or fill rock or soil to cap the carbonaceous material that will accumulate on the sea bottom.
The alga exists in nature broadly spread through out the ocean. You enable them to mass reproduce by adding the necessary trace minerals. They are easy to control by cutting off the flow of minerals.
If we are clever we might select a region of the ocean near the Sahara such that sand blows in anyway and we only need fill in the thin spots.
After a while (maybe sooner than you think) you could probably drill for oil there as our carbonaceous material cooks under the weight of ocean and sand and geothermal heat.
Nature does the hard work. This will take money, but a hell of a lot less than the cost of not using fossil fuels.
I thought of this over 10 years ago and posted it then. In part I am rewriting this in response to this below foolishness.
http://www.desmog.ca/2016/03/01/saudi-arabia-simply-sees-carbon-bubble-what-it
So to the basic facts in the recent past.
https://www.esrl.noaa.gov/gmd/ccgg/trends/full.html
From this website:
So around 1960 we had a CO2 ppm of under 320, and we now have a value of ~ 407, and the rate of rise is going up (because humans are increasing consumption of fossil fuels).
https://en.wikipedia.org/wiki/Carbon_dioxide_in_Earth%27s_atmosphere
From this above site, the ppm levels before the start of the industrial revolution were around 280 ppm. This below graph is from the above website.

~ 200 years of heavy industrialization and buildup of carbon-dioxide Now the ppm of carbon is ~ 407, and rising at about 2 ppm per year.
In 1950 the ppm of carbon was ballpark 300, I know it was slightly less in Thomas Jefferson's time and it was pretty flat for centuries before, and we had the year without a summer not long after that, so for the sake of argument call the amount we need to move the content of the atmosphere down to arrest global warming is 100 ppm, and that the rate of rise is now on the order of 2 ppm CO2 per year.
How are we going to do that I hear you ask? Like this, and note that this sort of thing has been done by nature from time to time so this is not "unnatural" and is in fact how many fossil fuel deposits formed.
We need to have a means of conversion that converts and sequesters a minimum equivalent of 2 ppm a year to match current addition of CO2. Double that would pull us back down to 300 ppm in 50 odd years, and 25 if we are cranking out 6 ppm worth per year. 2 for each year a to stay level, 4 per year reduces the ppm by 100 ppm per 25 years. If we started in 2020, by 2045 we are back to CO2 levels of 1950.
Now follows math, if you cannot deal with it, sorry. So how many tons of CO2 is 1 ppm in the atmosphere? I am going to omit sources of commonly known things like the radius of the earth and commonly known constants from physics and chemistry.
The radius of the earth is 6371 km, the surface area of a sphere is 4*PI*R^2
So the surface area of the earth is:
510 e+6 square kilometers or 510 e+14 square meters.
The atmosphere has a pressure of 14.7 psi at sea-level, thus a pressure of 10,334 kg per square meter.
The molecular weight of air is 28.97 grams/mol the molecular weight of CO2 is 44 grams/mol
So the weight of CO2 per ppm per square meter is: 10,334 *44/(28.97*1e6)= 0.0157 kg/m^2
Or 15.7 metric tons of CO2 per square kilometer per ppm of CO2 atmospheric content.
Total of 8 e+9 metric tons of CO2 per each ppm on earth. The carbon is a mass fraction is 12/44 of the mass of CO2
To get a handle on how much surface area of the earth we need for photosynthesis, I shall assume that we can get efficiency of production of carbon to sequester roughly equal to that of sugar cane conversion of sunlight+ CO2 to O2 + sucrose+ cellulose and other carbonaceous matter at a low latitude as that is where we place the farm.
http://www.ers.usda.gov/datafiles/Sugar_and_Sweeteners_Yearbook_Tables/US_Sugar_Supply_and_Use/Table15.xls
In Hawaii (low latitude) typical production rates of pure sugar per acre of land per year is 10 short (2000 lb) tons in that they have deducted seed and note that ~ 90-100 tons of raw cane are produced per acre. A square kilometer is 247.1 acres. Thus a square kilometer can produce 10*247.1/1.1 = 2246 metric tons per square kilometer per year of pure sugar, and about ten times that of total carbonaceous material.
Then sugar (sucrose - C22H22O11) has a molecular weight of 342.3 grams per mol. Of which 264/342.2 is carbon, assume for the sake of argument this is approximately correct all the carbonaceous material generated.
So to get 1 ppm of CO2 out of the air in one year I need to produce and sequester
8E+9 x 12/44 x 342.2/264 tons of pure sugar or 2.83 e+9 metric tons of sugar.
For which I need 1.260 e+6 square kilometers of which the earth has 510. E+6
To get 6 ppm per year, I would need to pull down the CO2 back to 1950 levels in 25 years from start I need 7.56 million square kilometers of sea near the equator. Again the earth has 510 million, this is about 1.5% of the earth's surface. A circle 3100 km in diameter will do it.
I can do better by using the full weight of carbonaceous material, rather than about 1/10th, that would reduce the required area to about 1/10 or 0.76 million square kilometers, or a circle of ~ 990 kilometers in diameter.
You should get the point, nothing about this is impossible. This is possible and not really expensive in the long haul, not compared to the alternative.
All we do is build buoys that spread fertilizer, and load them with fertilizer, and bring in periodic loads of sand or fill rock or soil to cap the carbonaceous material that will accumulate on the sea bottom.
The alga exists in nature broadly spread through out the ocean. You enable them to mass reproduce by adding the necessary trace minerals. They are easy to control by cutting off the flow of minerals.
If we are clever we might select a region of the ocean near the Sahara such that sand blows in anyway and we only need fill in the thin spots.
After a while (maybe sooner than you think) you could probably drill for oil there as our carbonaceous material cooks under the weight of ocean and sand and geothermal heat.
Nature does the hard work. This will take money, but a hell of a lot less than the cost of not using fossil fuels.
I thought of this over 10 years ago and posted it then. In part I am rewriting this in response to this below foolishness.
http://www.desmog.ca/2016/03/01/saudi-arabia-simply-sees-carbon-bubble-what-it


